A. ERDEMIR, G. R. FENSKE and R. A. ERCK
Argonne National Laboratory, Tribology Section, Materials and Components Technology Division,
Argonne, IL 60439 (U.S.A.). Printed in Surface and Coatings Technology, 43/44 (1990) 588—596
Abstract
An investigation was made of the formation and self-lubrication mechanisms of boric acid films on boric oxide coatings prepared by vacuum evaporation. Measured friction coefficients of a steel ball sliding on a boric oxide-coated steel disk and a sapphire ball sliding on a boric-oxide-coated alumina disk were 0.025—0.05 at steady state, depending on load and substrate material. This low friction was correlated with the formation of a lubricious boric acid film on boric oxide coatings exposed to open air.
For the mechanism of self-lubrication, the layered triclinic crystal structure of boric acid was proposed. The atoms constituting each boric acid molecule are arrayed in closely packed and strongly bonded layers that are 0.318 nm apart and held together by weak forces, such as van der Waals.
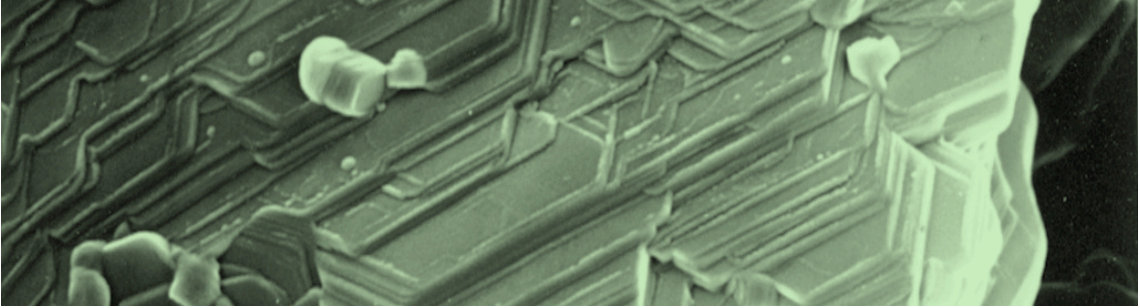
It is hypothesized that, during sliding, these layers can align themselves parallel to the direction of relative motion and, once so aligned, can slide over one another with relative ease to provide low friction. Structural and chemical findings were included to substantiate the proposed solid lubrication mechanism.
Conclusions
Under the test conditions of this study, low friction can be achieved on metallic and ceramic surfaces through the use of boric oxide coatings. Low friction is a direct consequence of the spontaneous formation of a boric acid film on boric oxide coatings exposed to open air. Friction coefficient decreases with increasing load and distance, and varies with substrate type.
Mechanistically, it is proposed that the low friction of boric acid is due to its layered triclinic crystal structure and unique bond characteristics. Under shear stresses, the layers can align themselves parallel to the direction of sliding motion; once so aligned, they can slide one over another, thus providing low friction.
Electron microscopy studies revealed plate-like crystallites with alignment parallel to the sliding surface; some microfeatures suggest that intercrystalline slip had occurred between the plate-like crystallites while sliding. Unlike MoS2, which provides low friction in non-humid environments, boric acid is formed in a humid environment and, as demonstrated in this study, can provide low friction in such environments.







